Molecules | Free Full-Text | Synthesis of 2-Oxazolines from Ring Opening Isomerization of 3-Amido-2-Phenyl Azetidines

Acid-Mediated Ring Expansion of 2,2-Disubstituted Azetidine Carbamates to 6,6-Disubstituted 1,3-Oxazinan-2-ones | Organic Letters

Anionic Ring-Opening Polymerization of N-(tolylsulfonyl)azetidines To Produce Linear Poly(trimethylenimine) and Closed-System Block Copolymers | Journal of the American Chemical Society
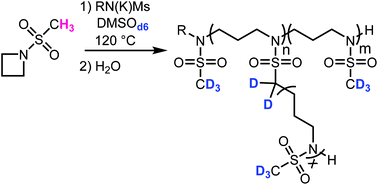
The anionic ring-opening polymerization of N-(methanesulfonyl)azetidine - Polymer Chemistry (RSC Publishing)

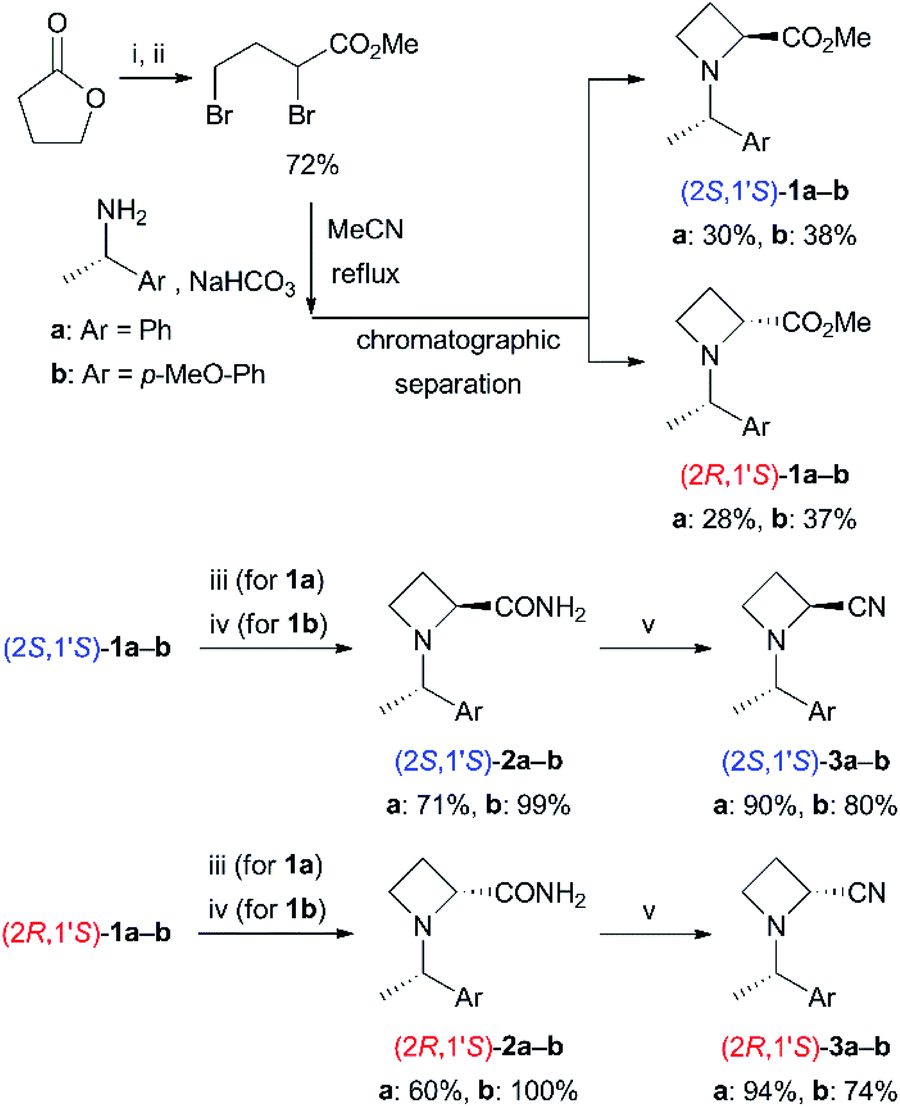
Chiral donor–acceptor azetines as powerful reactants for synthesis of amino acid derivatives | Nature Communications

Metabolism of Strained Rings: Glutathione S-transferase–Catalyzed Formation of a Glutathione-Conjugated Spiro-azetidine without Prior Bioactivation | Drug Metabolism & Disposition

Azetidines of pharmacological interest - Parmar - 2021 - Archiv der Pharmazie - Wiley Online Library

Aziridines and azetidines: building blocks for polyamines by anionic and cationic ring-opening polymerization - ScienceDirect

Ring‐Opening of Carbamate‐Protected Aziridines and Azetidines in Liquid Sulfur Dioxide - Lugiņina - 2016 - European Journal of Organic Chemistry - Wiley Online Library
![Molbank | Free Full-Text | Photoinduced Ring Opening of Methyl 1-Aryl-5-oxo-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazole-2-carboxylates in the Presence of Diaryl Disulfides Molbank | Free Full-Text | Photoinduced Ring Opening of Methyl 1-Aryl-5-oxo-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazole-2-carboxylates in the Presence of Diaryl Disulfides](https://www.mdpi.com/molbank/molbank-2023-M1670/article_deploy/html/images/molbank-2023-M1670-sch001.png)
Molbank | Free Full-Text | Photoinduced Ring Opening of Methyl 1-Aryl-5-oxo-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazole-2-carboxylates in the Presence of Diaryl Disulfides
Aziridines and azetidines: building blocks for polyamines by anionic and cationic ring-opening polymerization

Regio- and Stereoselective Ring Opening of Enantiomerically Enriched 2-Aryl Oxetanes and 2-Aryl Azetidines with Aryl Borates | The Journal of Organic Chemistry

Synthesis of azetidines by aza Paternò–Büchi reactions - Chemical Science (RSC Publishing) DOI:10.1039/D0SC01017K

The anionic ring-opening polymerization of N -(methanesulfonyl)azetidine - Polymer Chemistry (RSC Publishing) DOI:10.1039/C8PY00074C

Azetidiniums: Ring‐Expansion to Pyrrolidines, Piperidines, Azepanes, and Azocanes - Masson - 2020 - European Journal of Organic Chemistry - Wiley Online Library