Roche looks to delivery device to keep Lucentis biosimilars at bay - BioProcess InternationalBioProcess International

FDA Approves Lucentis 0.3 mg Prefilled Syringe | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology


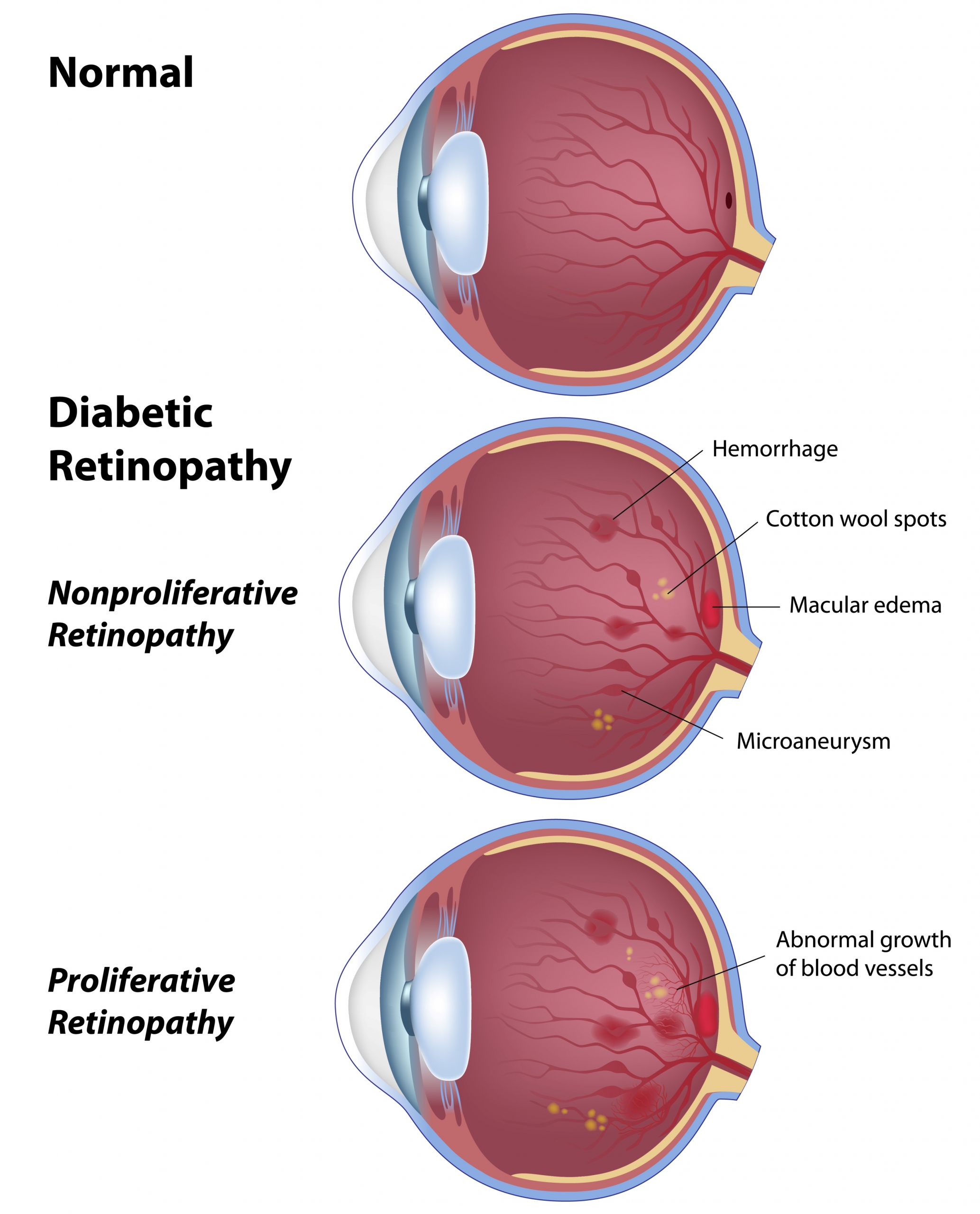

.jpg)