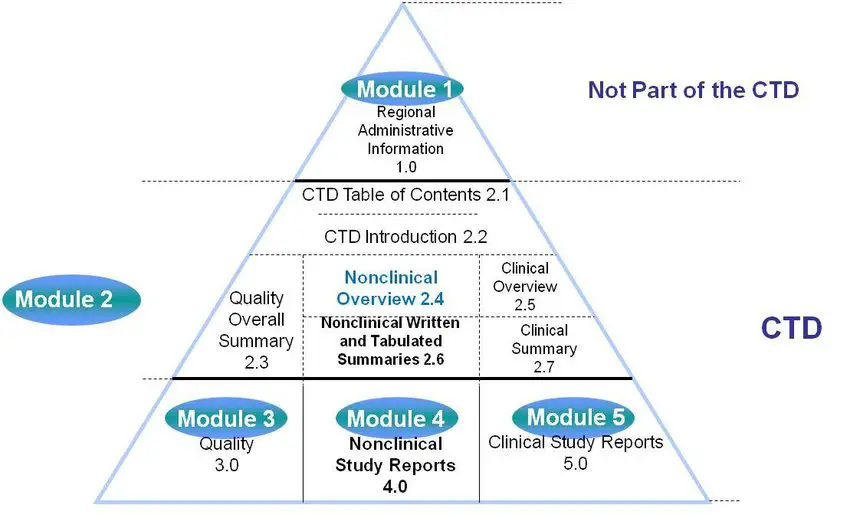
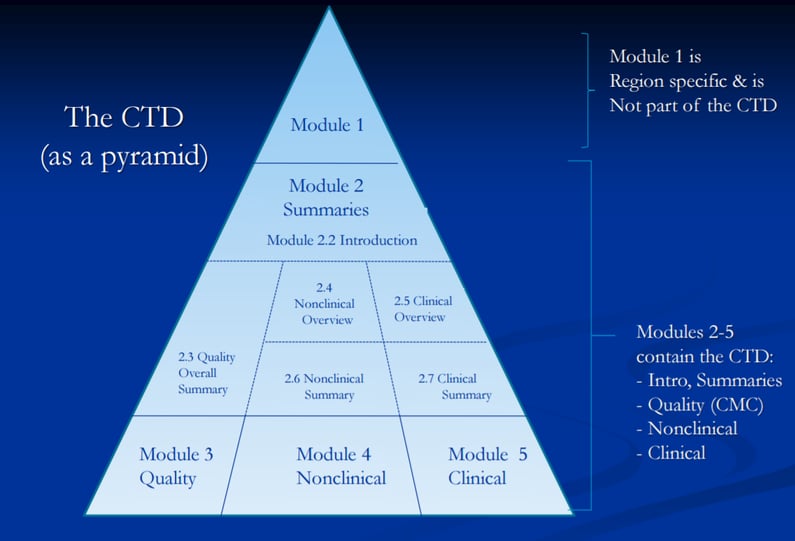
The Common Technical Document Elements (Source: ?Implementation of the... | Download Scientific Diagram

Regulation of Medical Devices Quiz - I) Module 1: FDA Organization and History The precursor to - Studocu

Best Practices for Submitting Promotional 2253 Submissions in the New Module 1 Specification - Sandra Krogulski, 2017