1 COMMON TECHNICAL DOCUMENT / ORIGIN OF CTD… ICH EWG CTD WAS OFFICIALLY SIGNED OFF IN NOVEMBER 2000, AT 5 TH ICH CONFERENCE; SAN DIEGO,CALIFORNIA. - ppt download

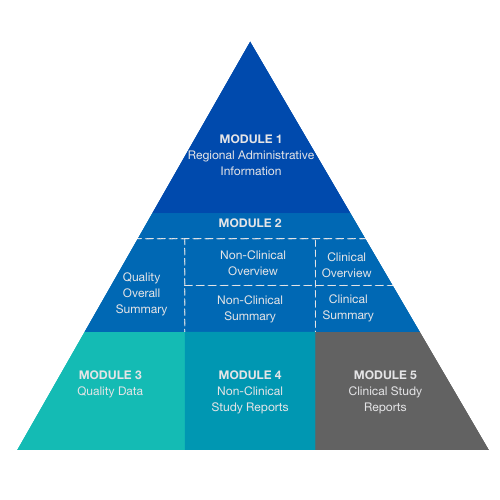
Schematic representation of the five modules in the Common Technical... | Download Scientific Diagram
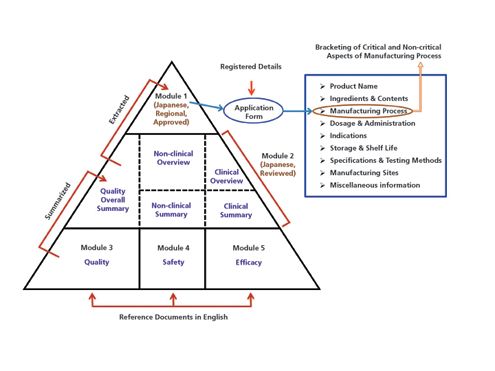
Exposé zum Thema: eCTD - Neue Wege der elektronischen Arzneimittelzulassung und die Vernetzung elektronischer regulatorischer P

A STUDY OF PROCEDURES FOR DOSSIER PREPARATION AND THEIR MARKETING AUTHORISATION IN DIFFERENT COUNTRIES OF SELECTED DRUG(S) | PharmaTutor

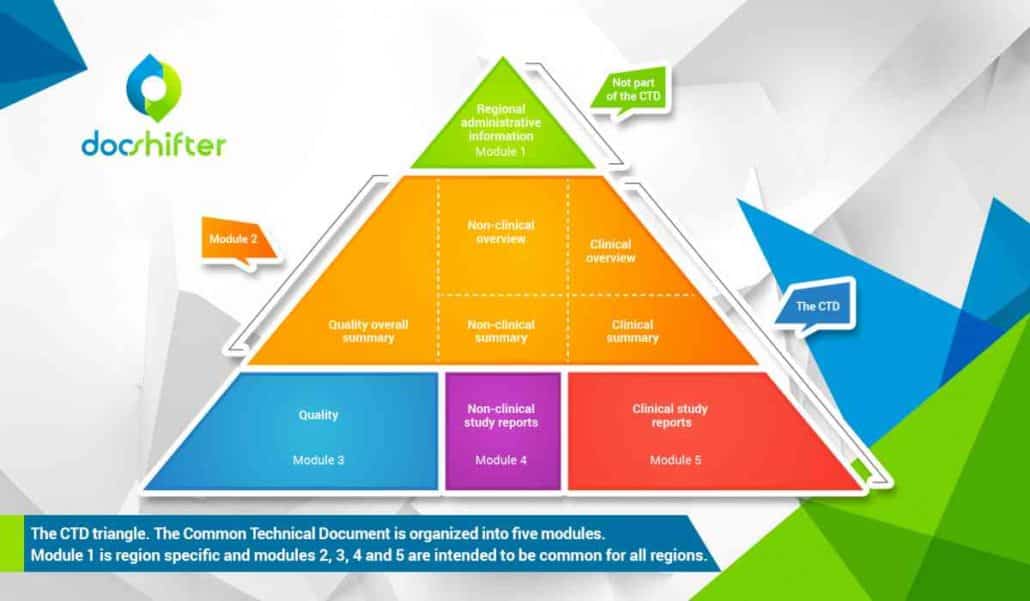
ClinProve - CTD Structure The Common Technical Document is divided into five modules: 1. Administrative and prescribing information 2. Overview and summary of modules 3 to 5 3. Quality (pharmaceutical documentation) 4.