Guideline on good pharmacovigilance practices (GVP) Module VI – Management and reporting of adverse reactions to medicinal pro

Revision 2 of EU Module VI of Guidelines on Good Pharmacovigilance Practices (GVP) - PharSafer® - Specialists in Global Clinical and Post Marketing Drug Safety

A NEW ERA OF DRUG SAFETY - NEW EU PHARMACOVIGILANCE (PV) LEGISLATION AND COMPARISON OF PV IN EU, US AND INDIA Review Article | Semantic Scholar

Establishing a Framework for the Use of Social Media in Pharmacovigilance in Europe | springermedizin.de

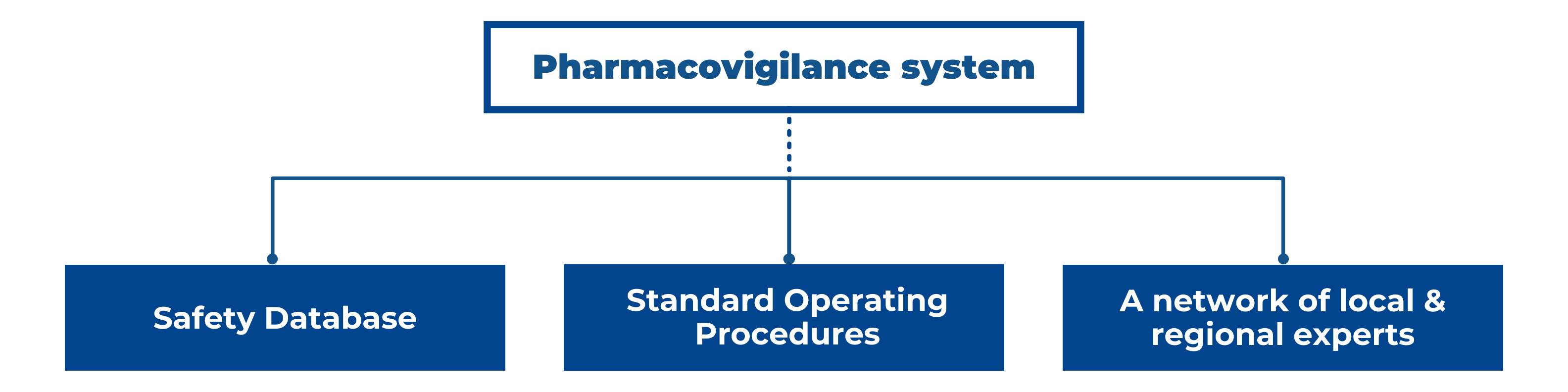
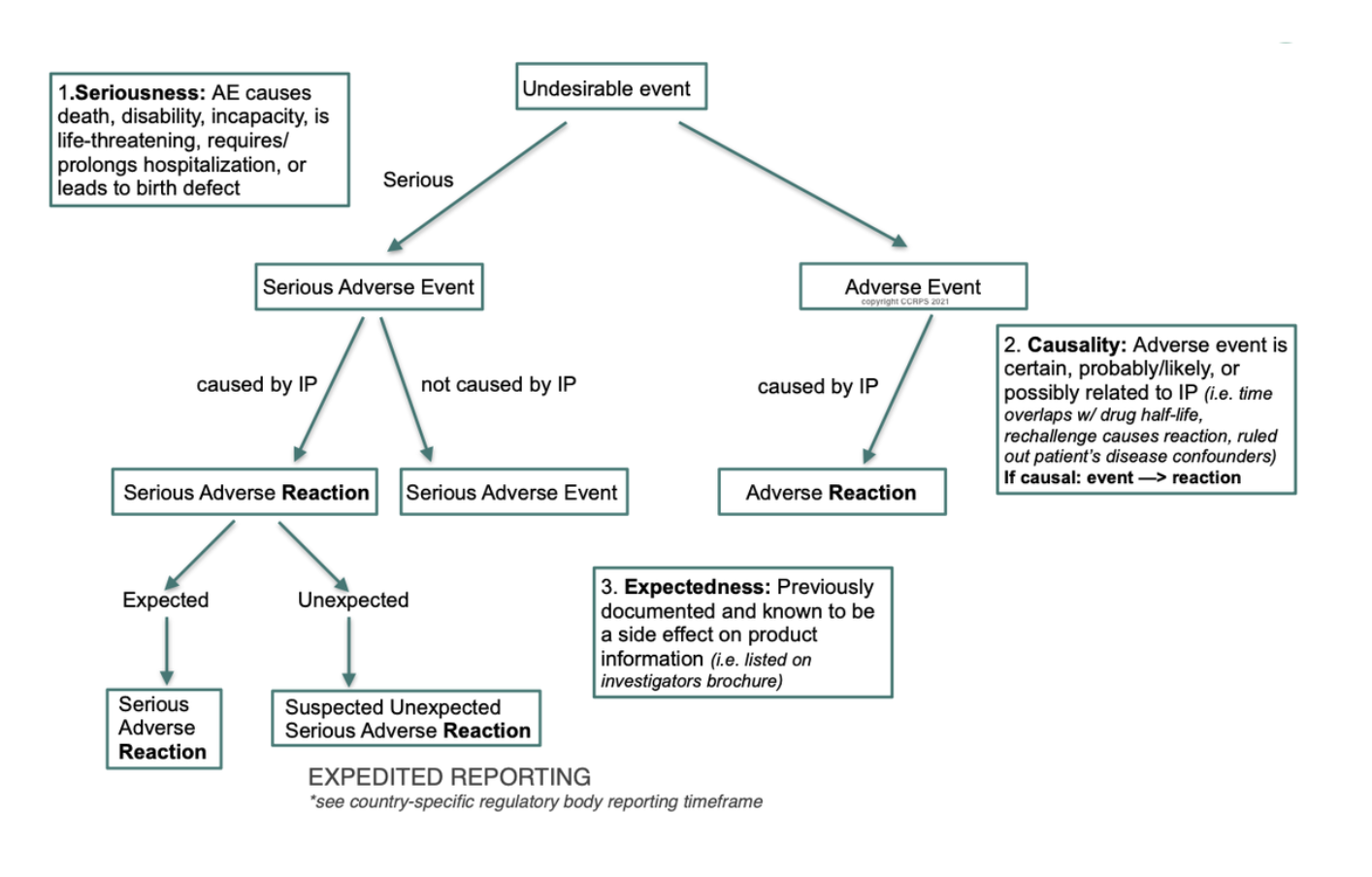
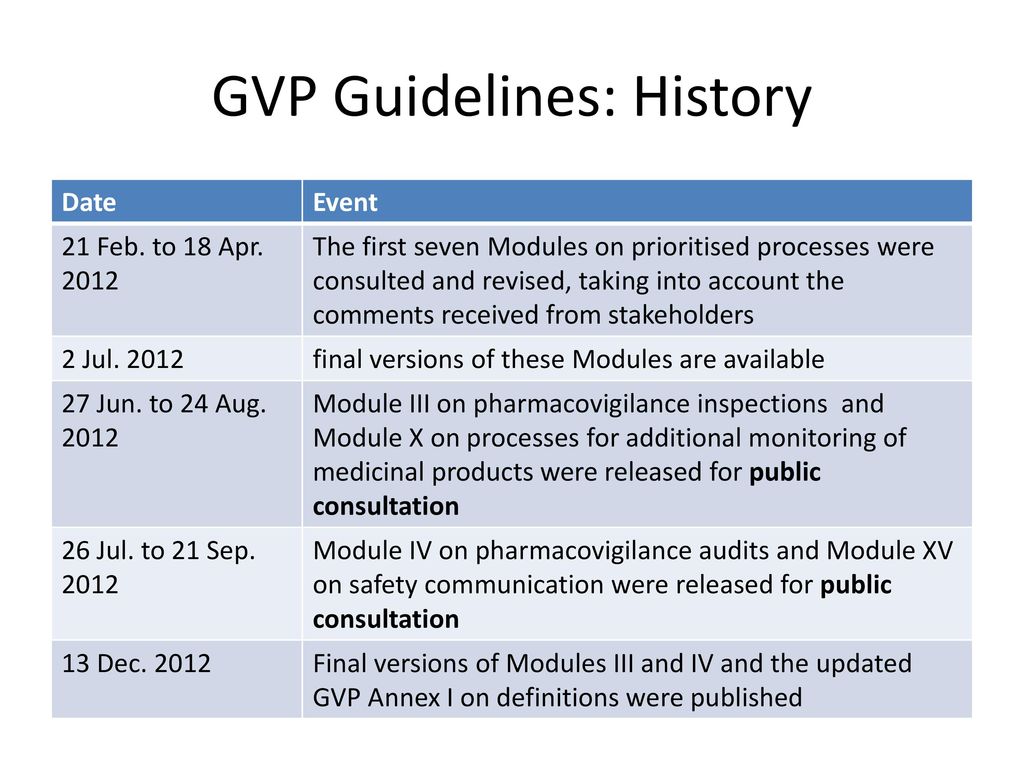
Eu2P Short Course: GVP Module VI - Collection, management and submission of reports of suspected ADR to Medicinal Products

Social Listening for Pharmacovigilance in Europe: Where are we in 2021? | Evolving our regulatory world together