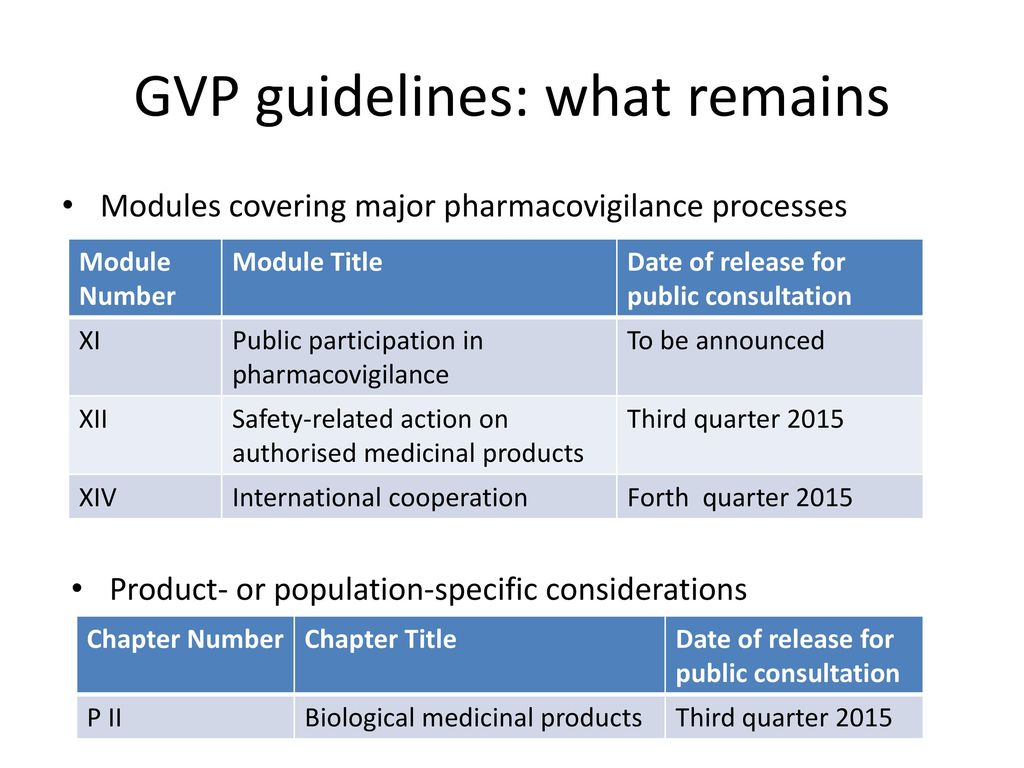
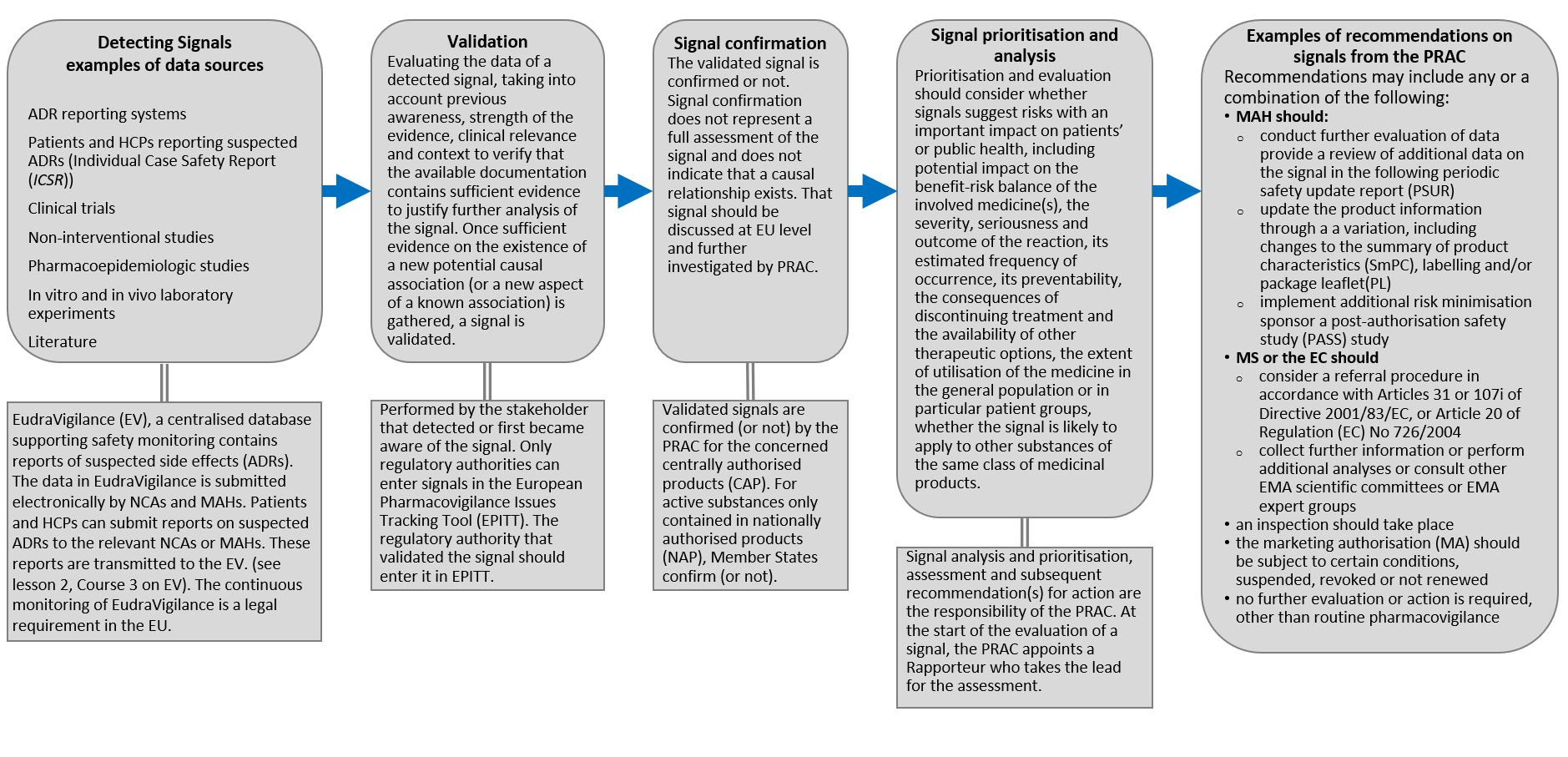
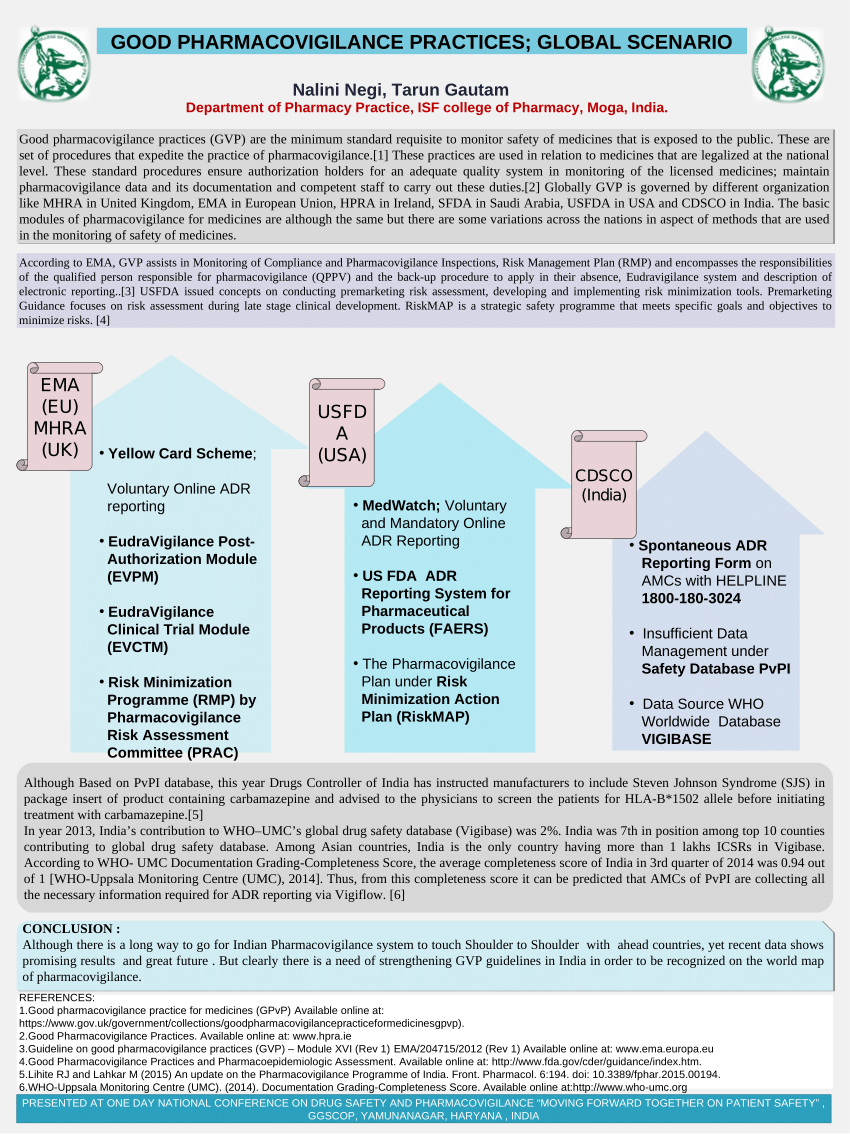
Eu2P Short Course: GVP Module VI - Collection, management and submission of reports of suspected ADR to Medicinal Products

Guideline On Good Pharmacovigilance Practices (GVP) Module VI - Management and Reporting of Adverse Reactions To Medicinal Products (Rev 1) | PDF | Pharmacovigilance | Adverse Effect