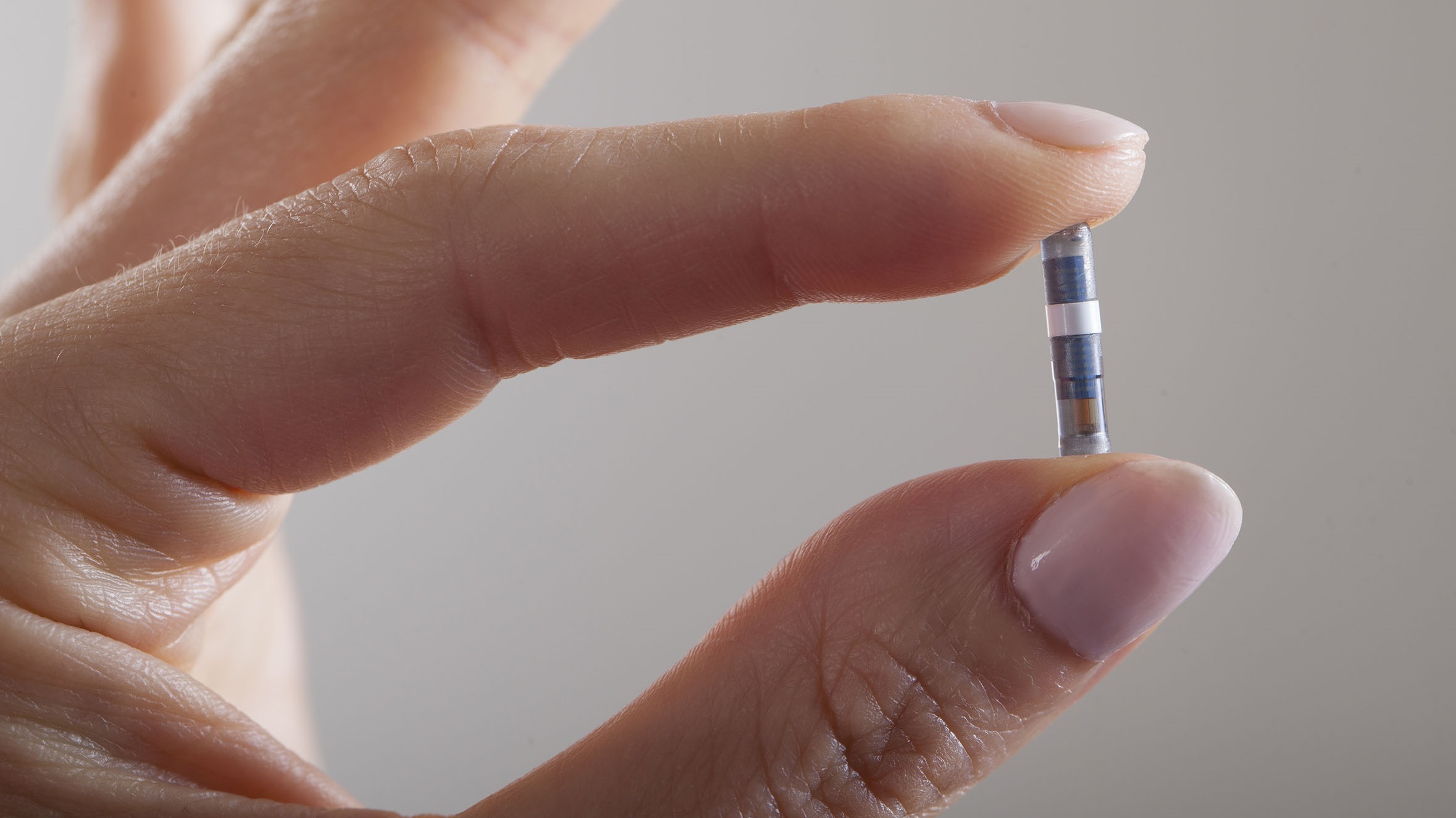
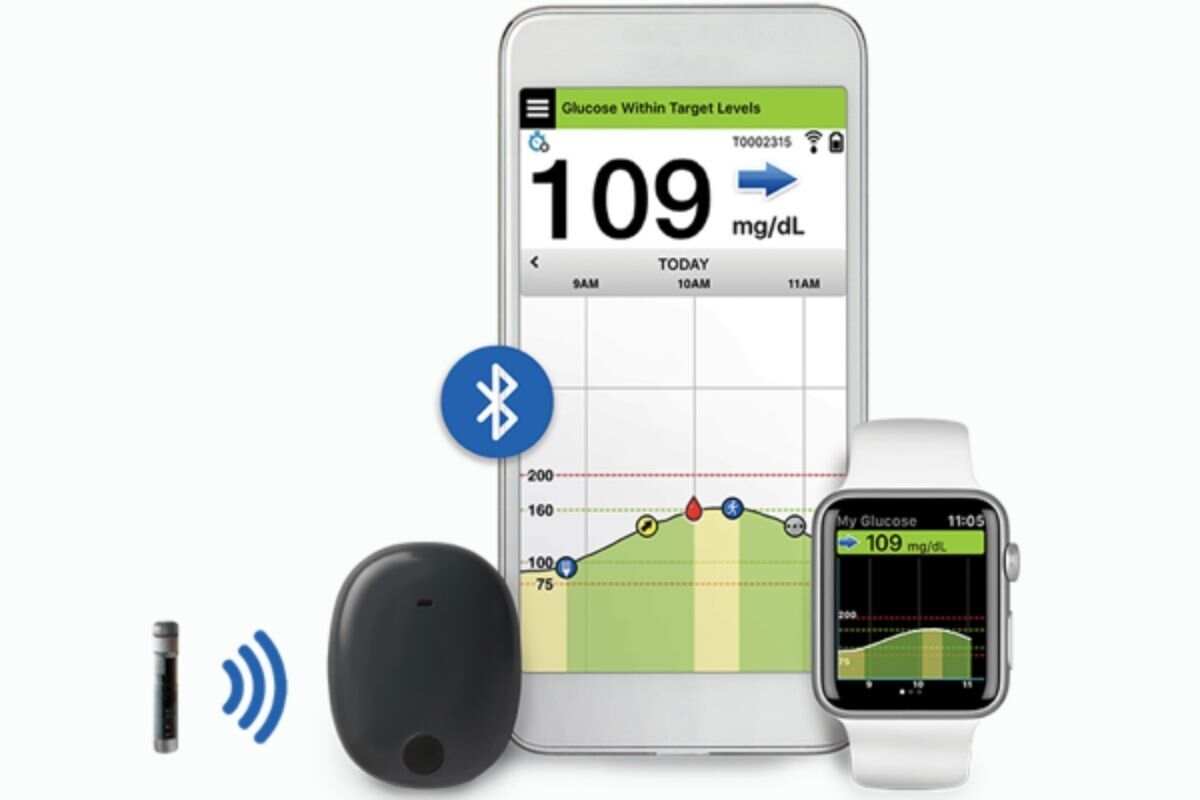
Diabetes Overview – Checking on promising tools from Dexcom, Bigfoot Biomedical and Senseonics | DeviceTalks
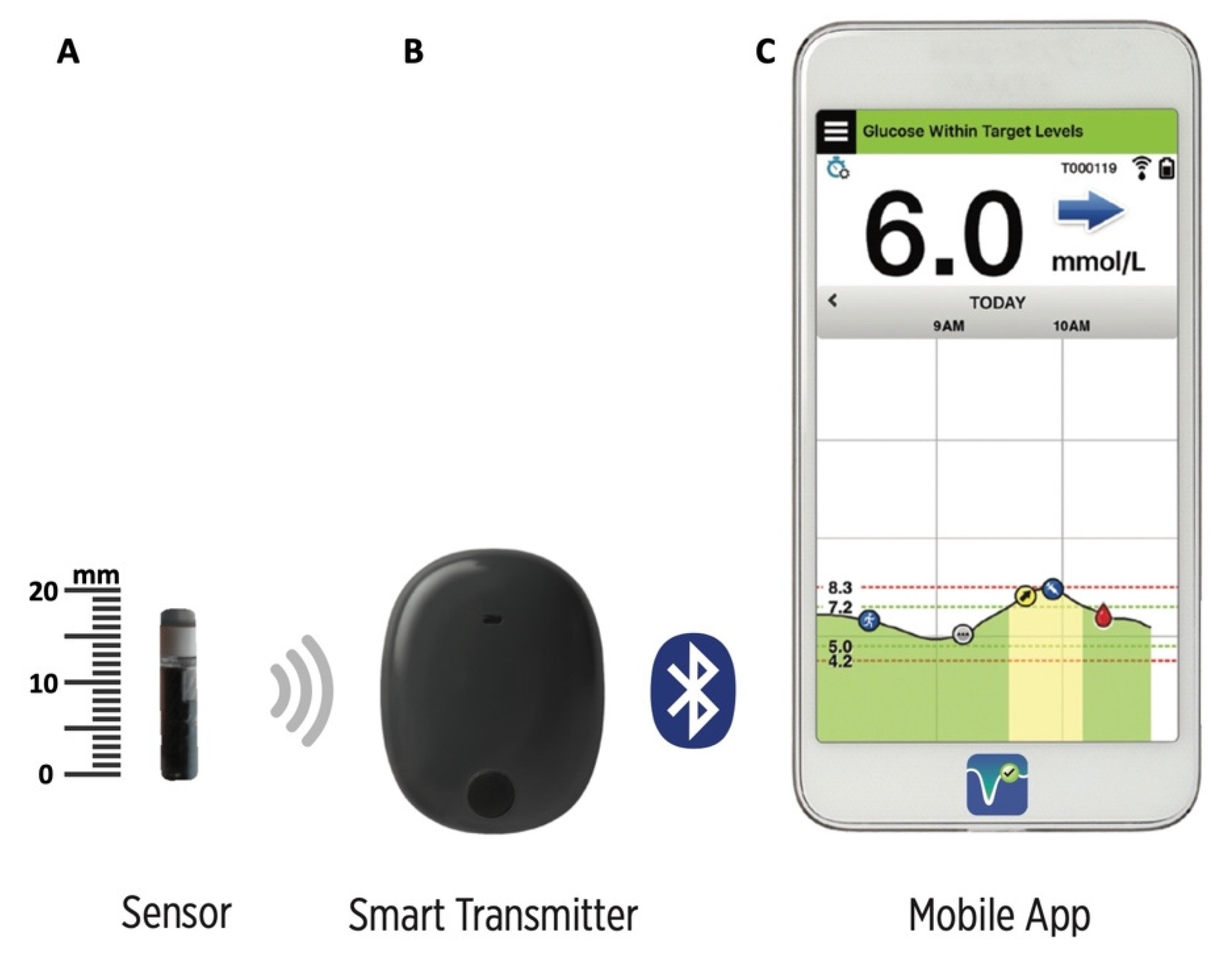
Animals | Free Full-Text | Clinical Use of a 180-Day Implantable Glucose Monitoring System in Dogs with Diabetes Mellitus: A Case Series

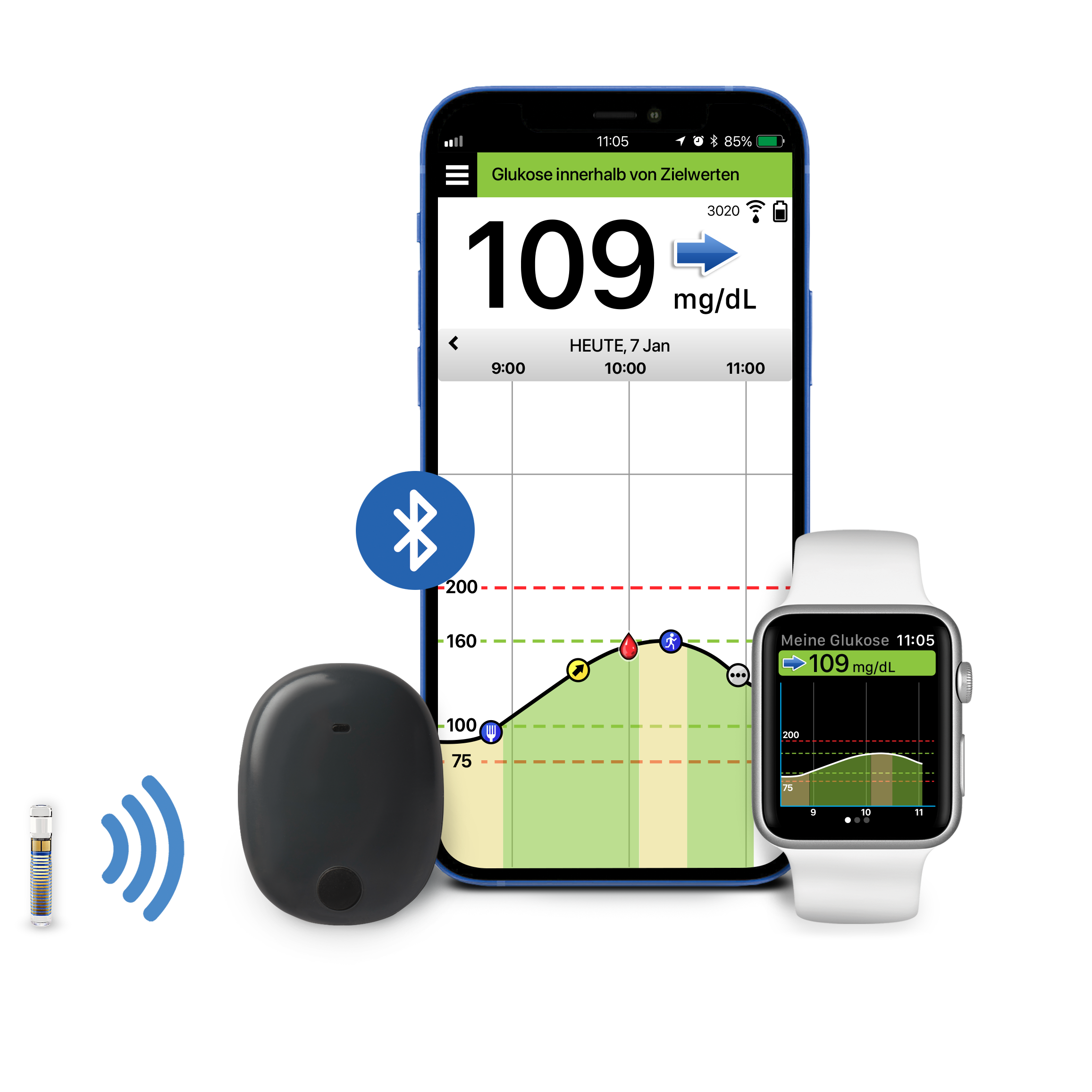
ASCENSIA DIABETES CARE KÜNDIGT GLOBALE INTEGRATION DES CGM-SYSTEMS VON EVERSENSE MIT APPLE HEALTH AN